Items we use every day come from steel- paperclips, tools, and cars to name a few. Sometimes you may wonder how those items are made, but have you ever wondered how the steel itself is made? Sir Henry Bessemer developed the first process for affordably manufacturing steel in 1856 called the Bessemer process. This process has become highly popular and has been adapted for mass production of steel. Different types of steel can be made, but it all starts with iron ore.


There are two different methods to make steel: basic oxygen and electric arc furnace.
Basic Oxygen Process (BOP)
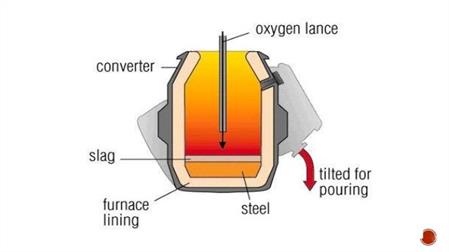
To charge the furnace, we start with scrap steel. It’s used as a coolant to control high temperatures produced by exothermic reactions between the blast-furnace iron and oxygen. About 25% will be from scrap and 75% will be liquid iron. Oxygen is then blasted through a lance that is lowered into molten metal. The oxygen combines with impure elements, carbon, silicon, manganese, and phosphorus to produce an exothermic reaction. Lime is then added to the furnace to help separate other impurities and turn them into slag. This stage lasts about 20 minutes, then a sample is taken for the composition of the metal. Now we want to run the steel out of a tap hole into a ladle to separate the slag from the steel. If any adjustments in the chemical composition are needed, then a secondary steelmaking process begins.
Electric Arc Furnace (EAF)
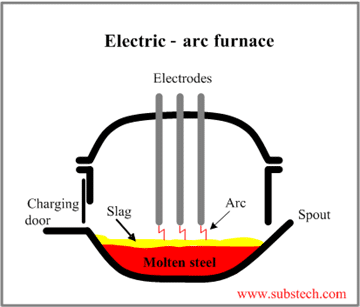
The electric arc method uses high-current electric arcs to melt steel scrap and convert it to liquid. We begin by loading scrap steel into the EAF from overhead. The lid contains electrodes which are lowered into the furnace after it’s been put into place. The electric current is then passed through electrodes to form an arc. Once the heat is generated by the arc, the scrap will melt. Other metals are added to the scrap to give the steel the chemical composition needed during this process. Once everything is melted, oxygen is blown into the furnace to purify the steel. Lime and fluorspar are added to combine with impurities. Before eliminating the slag, a sample is taken to check the chemical makeup. If the properties of the steel are good, the molten steel is poured into a ladle to be transported to the caster. This process typically takes about 90 minutes to make 150 tons.
Final Steps
Both processes end with the molten steel passing through casters and formed into slabs, blooms, and billets. These forms are the primary steel products and can be transformed into a wide range of finished products that are then used to create the products you’re familiar with. Slabs are rolled into flat products, such as plates. Blooms are shaped into structural shapes, like beams, and billets are formed into bars and rods.
